Consecutively occurring radiation-induced meningiomas with various pathologic diagnoses: a case report
Article information
Abstract
Meningioma is the second most common type of radiation-induced neoplasia that occurs after cranial radiotherapy. In this paper, we report a case of multiple radiation-induced meningiomas (RIMs) with different pathologies. A 23-year-old woman had a medical history of medulloblastoma at 2 years of age, for which she received chemotherapy and radiotherapy after operation. On follow-up magnetic resonance imaging (MRI) performed at the age of 13 years, RIM was observed in the left sphenoid ridge. The meningioma was treated with surgery and Gamma Knife radiosurgery. On follow-up MRI performed at 21 years of age, another RIM was found in the right temporal dura. The second RIM was stable after Gamma Knife surgery, but a third RIM occurred in front of the second. We removed the second and third RIMs in one operation. The pathological diagnoses of the first, second, and third RIMs were transitional, meningothelial, and atypical meningothelial meningioma, respectively. As shown in this case, RIM can develop several decades after exposure to radiation, and various grades of meningioma can occur at multiple sites. Therefore, patients who have undergone radiotherapy should receive long-term follow-up to check for RIM, and the appropriate treatment should be administered for the expected grade.
INTRODUCTION
Radiotherapy and radiosurgery play important roles in the treatment of brain tumors. However, radiation is closely related to several long-term complications, including leukoencephalopathy, vascular injury, arteritis, neuritis, hypothalamic-pituitary axis insufficiency, and radiation-induced neoplasia [1,2]. Among radiation-induced neoplasias, meningioma is the second most common tumor that can occur after cranial radiotherapy [3]. Radiosurgery is also not an exception to radiation-induced tumors [4,5].
Various cases of radiation-induced meningioma (RIM) have been reported since the first report of RIM in the radiotherapy field after radiotherapy for optic nerve glioma was reported in 1953. Additionally, RIM is known to have different histological characteristics and a poorer prognosis than sporadic meningiomas [6,7]. In this paper, we report a case of RIM with different pathologies that occurred consecutively after radiotherapy.
Ethical statements
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University College of Medicine (No. 2021-2692-001), and the requirement for the patient’s written consent was waived as it was a retrospective study.
CASE REPORT
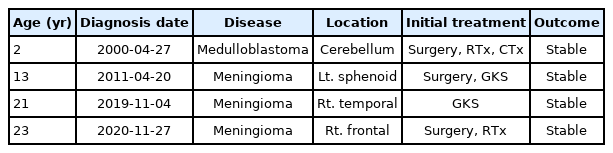
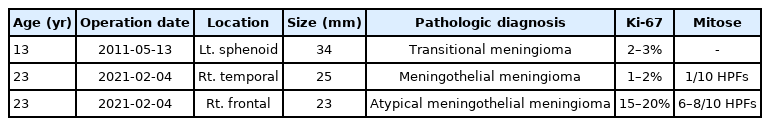
A 23-year-old woman presented with a growing mass in the right frontal dura. The patient had a medical record of medulloblastoma at 2 years of age. She had received a chemotherapy regimen consisting of vincristine, dactinomycin, cyclophosphamide, and doxorubicin after surgical resection, as well as whole-brain radiotherapy, with a total dose of 5,520 cGy. On follow-up magnetic resonance imaging (MRI) performed at 13 years of age, 11 years after combined treatment, medulloblastoma had not recurred (Fig. 1A). However, a left sphenoid ridge meningioma was observed (Fig. 1B). The meningioma presented as a large enhancing calcified mass invading the cavernous sinus and foramen ovale. The sphenoid meningioma was surgically removed. The pathological diagnosis was transitional meningioma, and 2–3% of tumor cells expressed Ki-67 (Fig. 2A, B). Gamma Knife surgery (GKS) was performed for the cavernous sinus remnant tumor (Fig. 3; marginal dose of 14 Gy at 50% isodose level).

Magnetic resonance imaging performed at 11 years of age, after surgical and radiation therapy for medulloblastoma. (A) There was no recurrence of medulloblastoma on a gadolinium-enhanced T1 image (arrow). (A, B) However, radiation-induced meningioma was observed on the left sphenoid ridge. (C, D) The sphenoid meningioma was surgically removed, but a remnant tumor in the cavernous sinus was observed on a postoperative gadolinium-enhanced T1 image. Gamma Knife surgery was performed to remove the cavernous sinus remnant tumor.

Hematoxylin and eosin (H&E) staining (A) and MIB-1 immunostaining (B) of the sphenoid meningioma at ×200 magnification. The pathological diagnosis was transitional meningioma, and 2–3% of tumor cells expressed Ki-67. H&E staining (C) and CD45-Ki67 dual staining (D) of the right frontal meningioma at ×200 magnification. The pathological diagnosis was atypical meningothelial meningioma. Histopathological examination revealed frequent mitoses (6–8/10 high-power fields [HPFs]), and immunohistochemical analysis revealed Ki-67 expression in 15–20% of tumor cells. H&E staining (E) and CD45-Ki67 dual staining (F) of the right temporal meningioma at ×200 magnification. The pathological diagnosis was meningothelial meningioma. Histopathological examination revealed frequent mitoses (1/10 HPFs), and immunohistochemical analysis revealed Ki-67 expression in 1–2% of tumor cells.

Gamma Knife surgery was performed on the remaining cavernous sinus remnant tumor after surgical removal of the left sphenoid meningioma (marginal dose of 14 Gy at a 50% isodose).
On follow-up MRI performed at 21 years of age, another RIM with a size of 22 mm was found in the right temporal dura. For temporal meningiomas, only GKS was performed (Fig. 4; marginal dose of 14 Gy at the 50% isodose level).

Magnetic resonance imaging performed 19 years after surgical and radiation therapy for medulloblastoma. There was no recurrence of medulloblastoma, and the left sphenoid meningioma was also stable (C; arrows). However, another radiation-induced meningioma was observed in the right temporal dura on a gadolinium-enhanced T1 image (A, B). Gamma Knife surgery was performed for the right temporal meningioma (D, E; marginal dose of 14 Gy at a 50% isodose).
On follow-up MRI performed 1 year after GKS, the treated right temporal dura meningioma was stable, but a newly developed meningioma was observed in the right frontal dura (Fig. 5). Since the growth rate of the right frontal meningioma was very high in the follow-up MRI performed 3 months later, surgery was performed instead of GKS. The right temporal meningioma was stable with GKS, but it was decided to be removed because it was very close to the right frontal meningioma and could therefore be removed at the same time. Tumors were extensively removed with the origin dura (Fig. 6; Simpson grade 1). The pathological diagnosis of a right frontal tumor was atypical meningothelial meningioma. Histopathological examination revealed frequent mitoses (6–8/10 high-power fields [HPFs]), and immunohistochemical analysis revealed Ki-67 expression in 15–20% of tumor cells (Fig. 2C, D). The pathological diagnosis of a right temporal tumor was a meningothelial meningioma. Histopathological examination revealed frequent mitoses (1/10 HPFs), and immunohistochemical analysis revealed Ki-67 expression in 1–2% of tumor cells (Fig. 2E, F). The postoperative clinical course was uneventful, and the patient showed no neurological deterioration. Approximately 3 months after the first surgery, a follow-up MRI showed that the right temporal and frontal meningiomas were well removed.

Magnetic resonance imaging performed 1 year after Gamma Knife surgery for right temporal radiation-induced meningioma (RIM). (A, B) The treated right temporal meningioma was stable, but a third RIM occurred in front of the second one (arrows). We removed the second and the third RIMs in one operation. Postoperative gadolinium-enhanced T1 images confirmed total removal (Simpson grade 1) of both RIMs (C, D).

Both the right temporal meningioma and the right frontal meningioma were removed in one operation. The tumors were extensively removed with their area of origin in the dura (Simpson grade 1).
The history of the patient’s disease, treatment, and outcomes are summarized in Table 1. The histopathological analyses of the removed RIM are summarized in Table 2.
DISCUSSION
The etiology of RIM cannot be clearly distinguished with sporadic meningioma, Cahan’s criteria may help define RIM. Cahan’s criteria were suggested in 1948, which were used to define a radiation-induced sarcoma [8,9]. And the modified Cahan’s standard used as the standard for RIM demonstration is as follows. (1) The lesion must be located an irradiated field. (2) A reasonable time interval, preferably longer than 4 years, must have elapsed between the initial irradiation and the new lesion. (3) The lesion must be histologically different from the previously original neoplasm. (4) The patient does not have a genetic predisposition for tumorigenesis. The patient should not have a genetic predisposition for tumor formation.
In this case, there were no medical record on the original radiation field of meningioma since the patient received radiation therapy 21 years ago. However, we believe that meningiomas could be included in the radiation field for medulloblastoma because the medulloblastoma of the patient was located in the high infratentorial area and the meningiomas were located in the low supratentorial area. Therefore, the authors judged that the meningiomas in this patient satisfies all four conditions required for RIM.
Since Mann et al. [10] first reported RIM in 1953, a large number of RIMs have been reported. According to reports, RIM has characteristics different from sporadic meningioma.
Histopathologic grade
The World Health Organization (WHO) classification divides meningiomas into three grades, according to histopathological diagnosis. It was previously reported that 84–91% of sporadic meningiomas were WHO grade I, 7–17% were WHO grade II, and 1–9% were WHO grade III meningiomas [11,12].
However, RIM has been reported to have a higher histopathological grade than sporadic meningiomas. Al-Mefty et al. [13] reported that 38% of patients who fulfilled the criteria for RIMs had atypical or malignant histopathological findings with a high recurrence rate. Yamanaka et al. [14] reported that out of 251 RIMs, 68.3% were WHO grade I, 26.8% were WHO grade II, and 4.9% were WHO grade III.
Due to its high histopathologic grade, RIM has a more aggressive clinical behavior and a higher recurrence rate than sporadic meningiomas.
Incidence rate according to age and gender
Most studies have shown a predominance of sporadic meningiomas in women. The incidence in females is approximately 2–3 times more than that in males. The incidence rate of sporadic meningioma progressively increases with age, peaking at 75–89 years [15,16].
The average age of patients who developed RIM after high-dose radiation was reported to be 30–38 years, and the average age of patients who developed RIM after low-dose radiation was reported to be 45–58 years [17,18]. However, unlike sporadic meningiomas, RIM has a clear exposure age of radiation, so it is reasonable to measure the latency period from radiotherapy rather than the average age of tumor diagnosis. Yamanaka et al. [14] reported, after the analysis of 251 cases of RIM, that the latency period between radiotherapy for primary lesions and the onset of meningiomas was 22.9±11.4 years. The latency period was shorter for patients who received high-dose radiation than for those who received low-dose radiation. They also found that systemic chemotherapy shortened the latency period of the RIM.
Multiplicity
RIM has an increased incidence of multiplicity. Strojan et al. [19] reported that 8% of 126 secondary meningiomas had multiple lesions after irradiation. Lillehei et al. [20] reported that multiple lesions were found in 16% of RIM cases.
There is no consensus regarding the treatment of RIM. We considered the tumor growth rate and expected pathological diagnosis when determining the treatment method for RIM in the patient. Through this case report, we can see that when multiple RIMs occur in the same patient, each RIM can have various pathological findings. Therefore, when deciding how to treat RIM, we should consider the fact that the characteristics of RIM differ from those of sporadic meningiomas.
CONCLUSION
As shown in this case, RIM can develop several decades after exposure to radiation, and various grades of meningiomas can occur at various sites. Therefore, patients who have undergone radiotherapy need follow-up for RIM for a long time, and appropriate treatment for the expected grade should be applied.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Authors’ contribution
In-Ho Jung: Writing - Original draft, Formal analysis, Data curation
Jun Yong Kim: Pathologic analysis
So Hee Park: Data curation
Won Seok Chang: Conceptualization, Resources, Supervision
Acknowledgements
The authors thank Eun Jung Kweon, RN, MSN, KOAPN, and Sang Keum Pak, RN, MSN for their tremendous help with data acquisition.
This work was supported by the Korea Medical Device Development Fund (Project Number: 202011D25).