Analysis of risk factors related to early post-traumatic seizures and post-traumatic epilepsy
Article information
Abstract
Objective
We investigated factors associated with early post-traumatic seizures (EPTSs) and post-traumatic epilepsy (PTE) to determine factors associated with PTE in comparison with previous reports.
Methods
We enrolled 78 patients with acute head trauma from January 2008 to December 2011 (range of follow-up duration, 5–12.9 years). We conducted a comparative analysis of various factors in both groups.
Results
Nine of the 78 patients experienced EPTSs during admission, and 18 patients developed PTE. All nine patients with EPTSs progressed to PTE. Treatment type, academic background, marital status, hemoglobin at hospital day 1 (HD#1), Glasgow coma scale (GCS) at admission, GCS at discharge, and the modified Rankin score (mRS) at the final visit were significantly different between the nine patients with EPTSs and the 69 patients who did not have EPTSs via the Mann-Whitney U-test. Statistically significant differences were identified in the type of brain trauma, treatment type, marital status, glucose level at HD#1, GCS at admission, GCS at discharge, and mRS at the final visit by the Mann-Whitney U-test between the 18 patients who developed PTE and the 60 patients who did not develop PTE.
Conclusion
The patients with PTSs and PTE were relatively younger and were more likely to be unmarried. It is premature to draw a firm conclusion, but more active administration and long-term meticulous follow-up may be needed for young, highly educated, unmarried male patients. In addition, the analysis of more patients over a longer period of time will be needed.
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of death and disability and plays a critical role in early post-TBI seizures within 1 week of assault, which could transpire in as many as 53% of all TBIs [1]. Post-traumatic seizures (PTSs) caused by TBI can occur either early (within 1 week of the injury) or late (after 1 week of the injury). Proper control of early post-traumatic seizures (EPTSs) is of paramount importance because seizure attacks in the acute stage can add secondary injury to the already damaged brain [2]. In the long run, approximately 25% of patients with a history of EPTSs will experience another episode in their later lives [2].
EPTSs are a strong predictor of late PTSs and epilepsy. In fact, up to 5% of all epilepsy cases and 20% of lesional epilepsy are post-traumatic [3]. In a previous study, seizures occurred in only 0.4% of adult patients admitted to hospitals with TBI, and association between in-hospital seizures and age, African-American ethnicity, obesity, hypertension, diabetes, history of myocardial infarction or cerebrovascular accident, cigarette smoking, and alcohol abuse was found [4]. The rate of severe TBI (Glasgow coma scale [GCS] score<9) was higher among the seizure group compared to the non-seizure group. Moreover, the seizure group had higher rates of complications including pneumonia, acute respiratory distress syndrome (ARDS), acute renal failure, pulmonary embolism, and increased intracranial pressure (ICP) during their hospital stay [4]. Identified risk factors of post-traumatic epilepsy (PTE) include chronic alcoholism, age of 65 years or older, penetrating injuries, traumatic intracranial hemorrhage (TICH), severity of injury, post-traumatic amnesia and/or loss of consciousness for more than one day, trauma-related focal neurological deficits, depressed skull fractures, cerebral contusions, and/or retained bone and metal fragments [5,6].
We conducted a retrograde analysis of patients who did not develop EPTSs and patients who developed PTE within the first 2 weeks of hospitalization. We also investigated patients with and without PTE during outpatient clinic follow-up. We attempted to identify factors related to the occurrence of EPTSs and PTE and to establish the risk factors to predict the need for antiepileptic drugs (AEDs) during outpatient follow-up due to concerns about PTE. We compared various possible factors associated with EPTSs and PTE.
MATERIALS AND METHODS
A total of 78 patients with only acute head trauma was subject to analysis from January 2008 to December 2011. The median follow-up duration was 7 years (range, 5–12.9 years). A total of 20 patients were female and 58 were male. The median age of analyzed patients was 45 years (range, 16 to 77 years). We only included patients with no other medical history. We tried to exclude all the medical factors of the patient and to check the influence of the patient’s social situation and the type of trauma at the time.
All patients were followed up for at least 5 years. The data were collected by a single tertiary center.
During the first 2 weeks of hospitalization, all patients were treated with sodium valproate via intravenous or oral administration as a first line AED that is thought to function as a GABAergic agonist to reduce neuronal excitability and prevent secondary brain injury from seizures. After 2 weeks, we changed oral AED treatment to carbamazepine, oxcarbazepine, or levetiracetam. In the outpatient clinic, we prescribed oral AEDs to patients who experienced seizure during a hospitalization period longer than 2 years. We did not prescribe AEDs to patients without seizure during the in-hospital period.
EPTSs are defined by their occurrence within 1 week of head trauma. These are acute symptomatic events and are not felt to represent epilepsy. PTE is a recurrent seizure disorder that apparently results from injury to the brain. This injury may be due to multiple types of head insults often labled TBI.
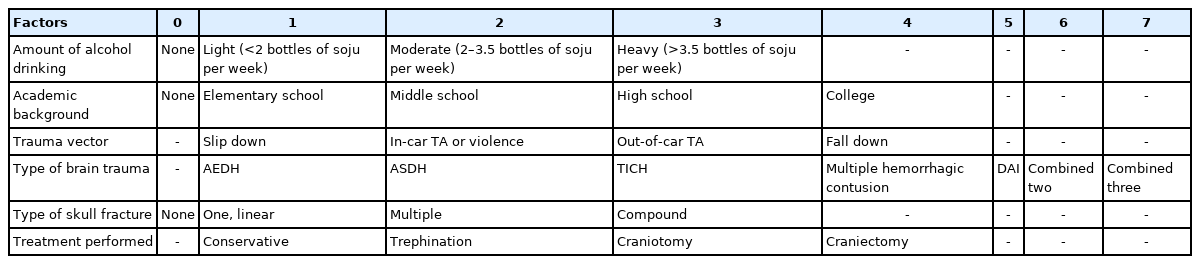
We analyzed sex, age, alcohol consumption, smoking, marital status, academic background, trauma vector, type of brain trauma, type of skull fracture, performed treatment, GCS at admission, blood glucose level (at admission, hospital day [HD] #1, HD#7, HD#14), white blood cell (WBC) number (at admission, HD#1, HD#7, HD#14), hemoglobin [Hb] (at admission, HD#1, HD#7, HD#14), seizure occurrence during hospitalization, GCS at discharge, and modified Rankin score (mRS) at the final visit. Alcohol consumption was graded as follows: none, 0; light, 1; moderate, 2; or heavy, 3. Academic background was graded as follows: no education, 0; elementary school, 1; middle school, 2; high school, 3; college or higher, 4. Trauma vector was classified as slip down, 1; in car traffic accident (TA) or violence, 2; out of car TA, 3; or fall, 4. Brain trauma type was graded as acute epidural hematoma, 1; acute subdural hematoma, 2; TICH, 3; multiple hemorrhagic contusion, 4; diffuse axonal injury, 5; two diseases combined, 6; or three diseases combined, 7. Skull fracture was scored as: no skull fracture, 0; one linear skull fracture, 1; multiple skull fractures, 2; or compound fracture, 3. Treatment was categorized as conservative, 1; trephination, 2; craniotomy, 3; or craniectomy, 4 (Table 1).
We analyzed two sets of two groups: patients with or without EPTSs and patients with or without PTE by Mann-Whitney U-tests using IBM SPSS vers. 23.0 software (IBM Corp., Armonk, NY, USA). We did not conduct multivariate analyses due to the small patient series.
Ethical statements
This study was approved by the Institutional Review Board (IRB) of the Jeonbuk National University Hospital (IRB No: 2013-10-022-002). Written informed consent was obtained from the patient.
RESULTS
Nine of 78 patients suffered from EPTSs during admission, and 18 of 78 patients developed PTE. All nine patients with EPTSs progressed to PTE. Among the 69 patients without EPTSs, nine developed PTE. We performed statistical analyses for all factors described in the Materials and Methods, and the results are shown in Table 2.
In the EPTSs group, significant differences in Mann-Whitney U-tests between the nine patients with EPTSs and the group of 69 patients who did not have EPTSs were identified in treatment type (p=0.020, 3.556 vs. 2.435), academic background (p=0.043, 3.111 vs. 2.337), marital status (p=0.015, 0.444 vs. 0.812), Hb HD#1 (p=0.007, 9.04 g/dL vs. 10.79 g/dL), GCS at admission (p=0.015, 8.3 vs. 11.48), GCS at discharge (p=0.006, 13 vs. 14.16), and mRS at last follow-up (p=0.005, 2.89 vs. 1.78). Mann-Whitney U-tests between the 18 patients who developed PTE and the 60 patients who did not develop PTE showed statistical difference in type of brain trauma (p=0.048, 5.89 vs. 5.03), treatment type (p=0.011, 3.28 vs. 2.35), marital status (p=0.015, 0.55 vs. 0.83), glucose level at HD#1 (p=0.031, 162.8 mg/dL vs. 112.6 mg/dL), GCS at admission (p=0.006, 8.88 vs. 12.78), GCS at discharge (p=0.018, 13.3 vs. 14.25), and mRS at the final visit (p=0.002, 2.6 vs. 1.7). Age (p=0.051), academic grade (p=0.059), and WBC at admission (p=0.055) showed a slight difference between the groups. Sex (p=0.110), type of skull fracture (p=0.071), trauma vector (p=0.208), amount of alcohol consumption (p=0.793), and smoking (p=0.670) did not show statistical differences (Table 2).
DISCUSSION
TBI affects nearly 1.4 million Americans annually [7], and PTSs affect approximately 15% of individuals who survive the initial ictus [8]. In our series, the rate of PTS occurrence was 13%.
PTSs are a cause of major complications after TBI and can increase the risk of PTE [9] through association with acute worsening of neurological status and poor clinical outcomes. EPTSs are defined as seizures that occur within the first week after trauma, and their incidence ranges between 2.1% and 16.9% in trauma patients [10]. EPTSs are a strong predictor of late PTSs and epilepsy. In fact, up to 5% of all epilepsy cases and 20% of lesional epilepsy are post-traumatic [3]. Prevention of EPTSs is thought to prevent PTE [11]. Some experimental studies have reported a permanent focus of abnormal activity leading to PTE syndrome [12]; therefore, brain trauma foundation guidelines recommend the use of anticonvulsants for prevention of EPTSs [12].
Early post-traumatic seizures
In a previous report, seizures occurred in only 0.4% of adult patients admitted to hospitals with TBI [4]. Inflammation, hemorrhage, edema, aberrant plasticity, and neurodegeneration all participate in the cerebral injury process. EPTSs are also a major cause for secondary brain injury through increasing cerebral blood flow, metabolic requirements, and ICP, which causes cerebral hypoxia and finally ischemia, resulting in elevated brain temperature and exacerbating indiscriminate neurotransmitter release [13]. Clinically, seizures correlate with patient age and severe conditions such as depressed skull fracture, intracranial hematoma, and penetrating head injury. Young children are more prone to early seizures, and adolescents and adults to late seizures [14]. A previous study reported an association between in-hospital seizures and age, African-American ethnicity, obesity, hypertension, diabetes, history of myocardial infarction and cerebrovascular accident, cigarette smoking, and alcohol abuse. The rate of severe TBI (GCS score<9) was higher among the seizure group compared to the non-seizure group. Moreover, the seizure group had higher rates of complications including pneumonia, ARDS, acute renal failure, pulmonary embolism, and increased ICP during hospital stay [15]. In addition, hemorrhage and skull fracture have been shown to increase inflammation and neuronal excitability, which effectively decreases the threshold for seizures [16].
In our series, the nine patients with PTSs were all male. The mean age was 42.3 years (range, 16–64 years), which is younger than the mean age for patients without PTSs (mean, 50.85 years). The PTS patient group had more complicated surgical treatment (mean, 3.556), higher academic grade (mean, 3.111), were unmarried (mean, 0.444), had lower Hb at the first day of admission (mean, 9.04 g/dL), lower GCS at admission (mean, 8.3), lower GCS at discharge (mean, 13), and higher mRS at the last follow-up (mean, 2.89) relative to the group of patients without PTSs.
Post-traumatic epilepsy
Epidemiological studies have found that PTE accounts for 10–20% of symptomatic epilepsy in the general population and 5% of all epilepsies [17]. Regarding PTE caused by war, the incidence is much higher in veterans than in civilian populations. The total incidence of PTE in the civilian population is approximately 2% [18], while that of the veteran population is as high as 25% when patients are followed for 5 or more years from the time of combat [14]. Moreover, the incidence of epilepsy ranges from 22–43% (median, 34%) 5 years after TBI in civilians, and the incidence is almost 50% at 10 or more years after injury for veterans [14,18]. Identified risk factors of PTE include chronic alcoholism, age 65 years or older, penetrating injuries, TICH, severity of injury, post-traumatic amnesia and/or loss of consciousness for more than 1 day, trauma-related focal neurological deficits, depressed skull fractures, cerebral contusion, and/or retained bone and metal fragments [2,16,17,19-21].
A critical determinant for PTE is TBI severity [22]. In a population-based clinical study (N=4,541) of TBI cases occurring between 1935 and 1984 in Olmstead County, MN, USA, the investigators found that the 5-year cumulative probability of unprovoked seizures was 0.7% in patients with mild TBI, 1.2% for moderate TBI, and 10.0% for severe TBI [6]. For the cohort with 30 years of follow-up, the cumulative incidence was 2.1% for mild TBI, 4.2% for moderate TBI, and 16.7% for severe TBI [6]. Older age can also increase the risk for PTE [21]. Gender overall does not appear to influence risk for PTE, although females can have a higher risk for PTE after milder injuries compared to males [15].
In our series, 18 patients with PTE showed a female to male ratio of 1:8. The mean age was 42.44 years (range, 16–70 years), younger than the patient group without PTE (mean, 53.6 years). The PTE group had more severe brain injury (mean, 5.89), more complicated surgical treatment (mean, 3.28), were unmarried (mean, 0.55), had higher glucose level at first hospital day (mean, 162.8 mg/dL), lower GCS at admission (mean, 8.88), lower GCS at discharge (mean, 13.3), and higher mRS at the last follow-up (mean, 2.6). Our data showed younger age (p=0.051) is a reliably significant risk factor of PTE.
In order to prevent permanent neurological sequela, current treatment of neurotrauma with regards to seizure development falls into one of two categories: prophylaxis for acute seizure or management of PTE, both of which have focused on the use of AEDs. To decrease the incidence of post-trauma seizures, the majority of clinicians is prescribing prophylactic medications for patients following head injury [23]. Prophylaxis is currently recommended for severe TBI by leading advisory boards (Brain Trauma Foundation and the American Academy of Neurology) for the first seven days [24]. While there is evidence that these prophylactic anticonvulsants reduce early seizures, there is no proven benefit for long-term prognosis [21]. Indeed, a meta-analysis of 10 randomized controlled trials showed a pooled relative risk reduction of 0.34 for early seizure prevention, indicating that 10 in every 100 patients will be seizure-free from treatment [25]. Thus, before prescribing these medications, consideration of injury severity, patient status, and side effect profile must be carefully reviewed [26]. In our series, six of nine patients with EPTSs and eight patients of 18 patients with PTE received anticonvulsant therapy more than 10 years.
Limitation
The limitation of this study is the small group size, which disallowed the ability to analyze each factor between the groups. It is necessary to enroll more patients in a different and/or expanded trial period with long term follow-up. The time to stop anticonvulsant treatments should be also considered further.
CONCLUSION
The differences between the results obtained from the analysis of patients included in this study are that patients with PTSs and PTE were of relatively younger age and were unmarried. In addition, it was confirmed that patients with EPTSs tended to have a high academic background and had low Hb at hospitalization, while patients with PTE had high glucose at hospitalization. Although there was no statistical significance, men experienced more PTSs and PTE than women. Because EPTS is the most important risk factor of PTE, it is necessary to investigate its relationships with young age and academic background. It is premature to conclude, but more active administration and long-term meticulous follow-up are needed for young, highly educated, unmarried male patients. In addition, the analysis of more patients over a longer period of time will be needed.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This paper was supported by the Fund of Biomedical Research Institute, Jeonbuk National University Hospital.